
Dating megalithic objects
To make sense of the ebb and flow of human history, we need to know the dates of events to put them into context. Dates are essential to historians and one of the first questions people ask of something like Stonehenge is how old it is.
With written history dating can be done by cross-referencing written records with others, but the stones stand mute - no written record attests to their antiquity, since they come from the time before our collective memories were written down in history. They are, by definition, pre-historic, and so alternative methods of establishing their age must be used.
The age must be derived from the physical characteristics of the objects, and where they are found in relation to other objects.
Stratigraphical analysis

Stratigraphical analysis uses the simple fact that common sense indicates that a deeper object must be older than an object buried at a shallower depth. This relies on the assumption that the ground has been built up in layers undisturbed by subsequent digging. That is not always the case - agriculture turns the soil over frequently, and over-enthusiastic archaeologists can also destroy precious evidence themselves by careless digging. In itself stratigraphical analysis can only give a relative timescale, though the presence of certain fossil remains and some kinds of pollen can tell us that objects must have been buried at some absolute time.
Where B is a particular type of animal only found in a particular time, then by comparing fossil remains at different sites, some picture of the relative ages of the sites can be drawn.
Radiocarbon Dating

The proportion of the unstable isotope of carbon, C14, gradually reduces with time. Knowing the relative amountrs of C12 to C14 originally present, the point on this graph corresponding to the measure relative amounts can be found, and the age of the item can be inferred.
A big breakthrough in determining absolute time came with the discovery of radioactivity. Radioactive elements become less radioactive with time as they decay into more stable non-radioactive forms of the element. Though it cannot be determined exactly when any individual atom is going to emit radiation and thus turn into a stable form (either directly or via intermediate stages), when a large number of atoms are present the time for the radioactivity to fall away to half its initial value can be measured, and is constant for that element. That period for the radioactivity to fall to half its initial value is called the half-life of that radioactive element.
Carbon, an essential constituent of all living things on Earth, has two forms - the stable form C12 and the unstable form C14, which is radioactive. That radioactivity decays by 50% in 5730+/-40 years. Living things continually renew their carbon from the environment, so while any organism is alive the balance of C12 and C14 is the same as that naturally present in the environment.Once the organism dies, the percentage of C14 falls. By measuring that proportion and working back from that percentage to when the proportion would be the same as that found in the environment, the age of the object can be inferred.
Three problems remain with radiocarbon dating. One is that it assumes that the proportion of unstable C14 to stable C12 has been constant in the environment, which may not be the case in modern times since Man has introduced some extra radioactivity into the environment.
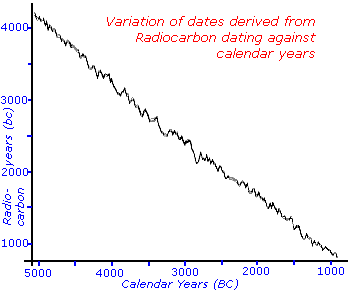
Nevertheless, as you can see from the graph of radiocarbon years assuming a linear decay versus the real calendar years the assumptions still hold fairly good. There are some times, like the years corresponding to 2100bc, which could give two distinct but closely spaced real equivalent dates since the graphs dips and comes up again.
The high precision of the measurements needed means any contamination of the object can bring forwards its dating by hundreds or thousands of years. Similarly any small experimental error can lead to large errors of the age derived from this method.
And finally, C14 dating can only be applied to organic remains - so direct application to stones is ruled out. Any human activity normally generates a lot of organic debris so this C14 dating can be applied to detritus left on site, but when we are talking of periods of thousands of years many organic remains decay and are often no longer to be found.
C14 dating involves burning a small piece of an archaeological find so it must be used with care, since that evidence is then lost to succeeding generations forever.
It cannot be applied to the stones directly, only to organic remains from the site. On a site like Stonehenge, which has gone through several phases of construction, it is difficult to tie together the organic remains exactly with particular stages of construction.
The decay of C14 is established from samples of bristlecone pines of California - trees that can be up to 5000 years old. The age of wood can be accurately determined to the year by counting the rings of the tree bark, a result of the annual spurts of growth in the Spring and slower growth in Winter.